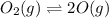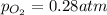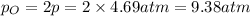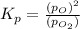Chemistry, 31.12.2019 04:31 angelespinosa521
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure of 4.97 atm, where it decomposes to o(g) by the reaction below.
o2(g) ⇄ 2 o(g)
at equilibrium, the partial pressure of o2 is 0.28 atm. calculate kp for this reaction at 4224 k.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2


Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure o...
Questions

Computers and Technology, 11.09.2019 19:10


English, 11.09.2019 19:10








English, 11.09.2019 19:10









Biology, 11.09.2019 19:10

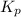 at 4224 K is 314.23.
at 4224 K is 314.23.