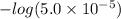Chemistry, 31.12.2019 04:31 James300102
Calculate the ph of a buffer solution prepared by dissolving 0.20 mole of cyanic acid (hcno) and 0.80 mole of sodium cyanate (nacno) in enough water to make 1.0 liter of solution. [k a(hcno) = 2.0 × 10 −4] 0.97 3.10 4.40 3.70 4.30

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

You know the right answer?
Calculate the ph of a buffer solution prepared by dissolving 0.20 mole of cyanic acid (hcno) and 0.8...
Questions

History, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01


History, 20.10.2020 18:01





Health, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

Advanced Placement (AP), 20.10.2020 18:01

Chemistry, 20.10.2020 18:01

Chemistry, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01



English, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01

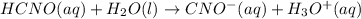
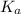 of HCNO is
of HCNO is 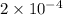 mol.
mol.![K_{a} = \frac{[CNO^{-}][H_{3}O^{+}]}{[HCNO]}](/tpl/images/0437/9975/6a3d8.png)
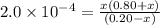
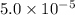 M
M ![-log[H_{3}O^{+}]](/tpl/images/0437/9975/74ef2.png)