Chemistry, 31.12.2019 02:31 yazanadel56
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbon-fluorine bond energy given that the c-c bond energy is 347 kj/mol, the c=c bond energy is 614 kj/mol, and the f-f bond energy is 154 kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbo...
Questions

Mathematics, 25.03.2020 21:51

Mathematics, 25.03.2020 21:51

Chemistry, 25.03.2020 21:51






Mathematics, 25.03.2020 21:51


Biology, 25.03.2020 21:51

English, 25.03.2020 21:51


Mathematics, 25.03.2020 21:51





History, 25.03.2020 21:51

![\Delta H_{rxn}=\sum [n_{i}\times (E_{bond})_{i}]-\sum [n_{j}\times (E_{bond})_{j}]](/tpl/images/0437/8404/d74f8.png)
 and
and  represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.
represents average bond energy in breaking "i" th bond and forming "j" th bond respectively. and
and 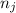 are number of moles of bond break and form respectively.
are number of moles of bond break and form respectively.