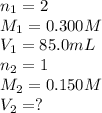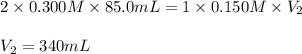Chemistry, 31.12.2019 02:31 htorres2p8urw0
How many milliliters of 0.150 m naoh are required to neutralize 85.0 ml of 0.300 m h2so4 ? the balanced neutralization reaction is: h2so4(aq)+2naoh(aq)→na2so4(aq)+2h2o (l).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
How many milliliters of 0.150 m naoh are required to neutralize 85.0 ml of 0.300 m h2so4 ? the bala...
Questions




Mathematics, 20.02.2020 03:35

Mathematics, 20.02.2020 03:35

Mathematics, 20.02.2020 03:35


Mathematics, 20.02.2020 03:35



Mathematics, 20.02.2020 03:35


Social Studies, 20.02.2020 03:35

Mathematics, 20.02.2020 03:36


Social Studies, 20.02.2020 03:36




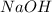 required to neutralize is, 340 mL
required to neutralize is, 340 mL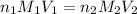
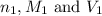 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 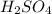
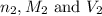 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.