
Chemistry, 31.12.2019 00:31 mayamabjishovrvq9
Determine the heat of reaction (δhrxn) for the reaction of calcium carbonate (caco3) with hcl to produce co2 by using heat of formation data:
caco3 (s) + 2 hcl (g) → cacl2 (s) + co2 (g) + h2o (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Determine the heat of reaction (δhrxn) for the reaction of calcium carbonate (caco3) with hcl to pro...
Questions

Mathematics, 19.10.2019 11:00





Mathematics, 19.10.2019 11:00


Mathematics, 19.10.2019 11:00

Mathematics, 19.10.2019 11:00

Social Studies, 19.10.2019 11:00

Biology, 19.10.2019 11:00

Mathematics, 19.10.2019 11:00


Biology, 19.10.2019 11:00





Advanced Placement (AP), 19.10.2019 11:00

Mathematics, 19.10.2019 11:00

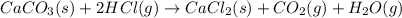
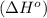 .
.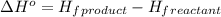
![\Delta H^o=[n_{CaCl_2}\times \Delta H_f^0_{(CaCl_2)}+n_{CO_2}\times \Delta H_f^0_{(CO_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{CaCO_3}\times \Delta H_f^0_{(CaCO_3)+n_{HCl}\times \Delta H_f^0_{(HCl)}]](/tpl/images/0437/6894/d865d.png)
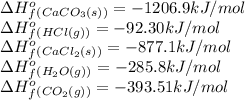
![\Delta H^o_{rxn}=[(1\times -877.1)+(1\times -393.51)+(1\times -285.8)]-[(1\times -1206.9)+(2\times -92.30)]=-164.9kJ](/tpl/images/0437/6894/ee64d.png)


