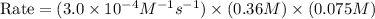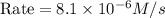Chemistry, 31.12.2019 00:31 texas101st78
The rate constant for the reaction nh4+(aq) + no2−(aq) --> n2(g) + 2h2o(l) is, k = 3.0 × 10^−4 m^-1 · s^-1. calculate the rate of the reaction if [nh4+] = 0.36 m and [no2−] = 0.075 m. (the reaction is in first order in regards to nh4+ as well as no2-) answer in scientific notation

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1


Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
The rate constant for the reaction nh4+(aq) + no2−(aq) --> n2(g) + 2h2o(l) is, k = 3.0 × 10^−4 m...
Questions





Chemistry, 20.04.2020 20:12



History, 20.04.2020 20:12


Health, 20.04.2020 20:12

Mathematics, 20.04.2020 20:12


Social Studies, 20.04.2020 20:12

History, 20.04.2020 20:13


English, 20.04.2020 20:13

History, 20.04.2020 20:13

Mathematics, 20.04.2020 20:13

English, 20.04.2020 20:13

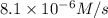
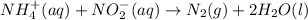
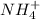 and
and 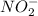 are the reactants.
are the reactants.![\text{Rate}=k[NH_4^+][NO_2^-]](/tpl/images/0437/6898/ed258.png)
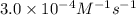
![[NH_4^+]](/tpl/images/0437/6898/5c46c.png) = concentration of
= concentration of ![[NO_2^-]](/tpl/images/0437/6898/10a69.png) = concentration of
= concentration of