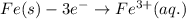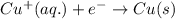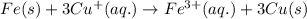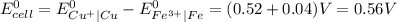Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction involving the galvanic cell made from a half-cell consisting of a copper electrode in 1 m copper(i) nitrate solution and a half-cell consisting of an iron electrode in 1 m iron(iii) nitrate. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number coefficients.) overall reaction

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction invol...
Questions

Mathematics, 25.09.2021 15:30

Mathematics, 25.09.2021 15:30


Physics, 25.09.2021 15:30

Mathematics, 25.09.2021 15:30



Mathematics, 25.09.2021 15:30

Computers and Technology, 25.09.2021 15:30

History, 25.09.2021 15:30

Computers and Technology, 25.09.2021 15:30

English, 25.09.2021 15:30

Mathematics, 25.09.2021 15:30

Computers and Technology, 25.09.2021 15:30





Social Studies, 25.09.2021 15:30

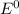 ) are given below-
) are given below-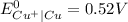
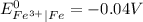
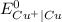 is greater than
is greater than 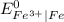 therefore Fe will be oxidized to
therefore Fe will be oxidized to 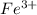 and
and 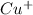 will be reduced to Cu
will be reduced to Cu