
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) 2 hi (g), are [h2] = 0.106 m; [i2] = 0.022 m; [hi] = 1.29 m calculate the new equilibrium concentration of hi (in m) if the equilibrium concentrations of h2 and i2 are 0.95 m and 0.019 m respectively.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2

Chemistry, 23.06.2019 13:30
Which of the following is true regarding chemical and nuclear reactions?
Answers: 1

Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1

Chemistry, 23.06.2019 15:30
If the theoretical yield of a reaction is 26.0 grams and you actually recovered 22.0 grams, what is the percent yield?
Answers: 2
You know the right answer?
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) ...
Questions





SAT, 18.04.2021 05:10


Mathematics, 18.04.2021 05:10


History, 18.04.2021 05:10



Mathematics, 18.04.2021 05:10



Geography, 18.04.2021 05:10


Mathematics, 18.04.2021 05:10

English, 18.04.2021 05:10


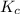 ) for the given chemical reaction, is given by the equation:
) for the given chemical reaction, is given by the equation:![K_{c} = \frac {[HI]^{2}}{[H_{2}]\: [I_{2}]}](/tpl/images/0435/6174/f8601.png)

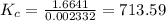
![K_{c} = \frac {[HI]^{2}}{(0.95\: M) \times (0.019\: M)}](/tpl/images/0435/6174/d2511.png)
![\Rightarrow K_{c} = 713.59 = \frac {[HI]^{2}}{0.01805}](/tpl/images/0435/6174/fef3c.png)
![\Rightarrow [HI]^{2} = 713.59 \times 0.01805 = 12.88](/tpl/images/0435/6174/d4ab1.png)
![\Rightarrow [HI] = \sqrt {12.88} = 3.589 M](/tpl/images/0435/6174/5120a.png)


