Chemistry, 28.12.2019 05:31 viktoria1198zz
At a particular temperature the first-order gas-phase reaction n 2o 5→ 2no 2 + (1/2)o 2 has a half-life for the disappearance of dinitrogen pentoxide of 5130 s. suppose 0.450 atm of n 2o 5 is introducted into an evacuated 2.00 l flask. what will be the total gas pressure inside the flask after 3.00 hours? a. 0.969 atm b. 0.105 atm c. 0.795 atm d. 1.14 atm e. 0.864 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
At a particular temperature the first-order gas-phase reaction n 2o 5→ 2no 2 + (1/2)o 2 has a half-l...
Questions

Advanced Placement (AP), 11.12.2020 01:00



English, 11.12.2020 01:00

Geography, 11.12.2020 01:00

Law, 11.12.2020 01:00

Engineering, 11.12.2020 01:00


Mathematics, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00


Health, 11.12.2020 01:00



English, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00




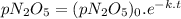
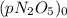 is the initial pressure
is the initial pressure