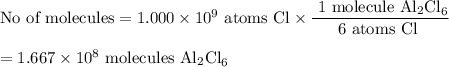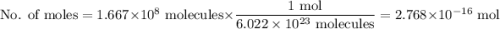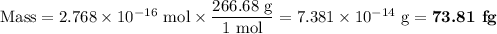Chemistry, 28.12.2019 05:31 giulianna41
Calculate the mass of gold(iii) chloride au2cl6 that contains a billion 1.000x10^9 chlorine atoms.
be sure your answer has a unit symbol if necessary, and round it to 4 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
Calculate the mass of gold(iii) chloride au2cl6 that contains a billion 1.000x10^9 chlorine atoms.
Questions



Mathematics, 21.03.2020 02:16





Mathematics, 21.03.2020 02:16

Mathematics, 21.03.2020 02:16


History, 21.03.2020 02:16



Chemistry, 21.03.2020 02:16


Mathematics, 21.03.2020 02:16

History, 21.03.2020 02:16


Mathematics, 21.03.2020 02:16

History, 21.03.2020 02:16