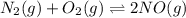Chemistry, 28.12.2019 04:31 narutoxptheninja
For the reactionn2(g)+o2(g)? 2no(g)classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the reaction. a) half oxygenb) double oxygenc) double nitrogend) half nitrogene) double nitrogen monoxidef) half nitrogen monoxide

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
For the reactionn2(g)+o2(g)? 2no(g)classify each of the following actions by whether it causes a lef...
Questions





Spanish, 29.06.2019 00:00




Social Studies, 29.06.2019 00:00


Biology, 29.06.2019 00:00



Chemistry, 29.06.2019 00:00

Biology, 29.06.2019 00:00

Physics, 29.06.2019 00:00


Social Studies, 29.06.2019 00:00


History, 29.06.2019 00:00