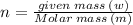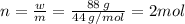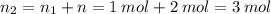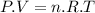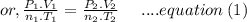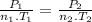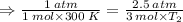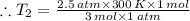Chemistry, 28.12.2019 04:31 hilljade45
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.0 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.5 atm. what is the final temperature? assume the solid carbon dioxide takes up negligible volume.
a. 150 k
b. 200 k
c. 250 k
d. 300 k
e. 400 k

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide....
Questions


Computers and Technology, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

History, 20.01.2021 20:40

English, 20.01.2021 20:40



Health, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

Geography, 20.01.2021 20:40


Mathematics, 20.01.2021 20:40




History, 20.01.2021 20:40

English, 20.01.2021 20:40