
Chemistry, 28.12.2019 02:31 appattuvilai1234
Solid copper (ii) oxide is placed in a sealed container with an excess of ammonia gas. when equilibrium is established the partial pressure of n2 is 0.255atm. the reaction is as follows: 3cuo (s) + 2 nh3 (g) ↔ 3 cu (s) + n2 (g) + 3 h2o (g) a) what is the partial pressure of h2o (g) at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
Solid copper (ii) oxide is placed in a sealed container with an excess of ammonia gas. when equilibr...
Questions

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50


Biology, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

Chemistry, 12.12.2020 15:50

English, 12.12.2020 15:50

Computers and Technology, 12.12.2020 15:50


Mathematics, 12.12.2020 15:50




Mathematics, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



Social Studies, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

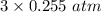 = 0.765 atm
= 0.765 atm

