Consider the following standard heats of formation:
p₄o₁₀(s) = -3110 kj/mol
h₂o(l) =...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Questions

Mathematics, 19.03.2020 23:25

Mathematics, 19.03.2020 23:25










Mathematics, 19.03.2020 23:26





Mathematics, 19.03.2020 23:26



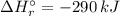
![\Delta H_{f}^{\circ } [P_{4}O_{10}(s)]](/tpl/images/0435/2502/5b047.png) = -3110 kJ/mol,
= -3110 kJ/mol,![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/2502/46f61.png) = -286 kJ/mol,
= -286 kJ/mol, ![\Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]](/tpl/images/0435/2502/1d826.png) = -1279 kJ/mol
= -1279 kJ/mol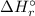 = ?
= ?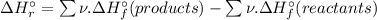
![\Delta H_{r}^{\circ } = [4 \times \Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]] - [1 \times \Delta H_{f}^{\circ } [P_{4}O_{10}(s)] + 6 \times \Delta H_{f}^{\circ } [H_{2}O(l)]]](/tpl/images/0435/2502/c9ebf.png)
![\Rightarrow \Delta H_{r}^{\circ } = [4 \times (-1279\, kJ/mol)] - [1 \times (-3110\, kJ/mol) + 6 \times (-286\, kJ/mol)]](/tpl/images/0435/2502/ea7a2.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-3110\, kJ -1716\, kJ]](/tpl/images/0435/2502/7e6ce.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-4826\, kJ] = -290\,kJ](/tpl/images/0435/2502/ee612.png)


