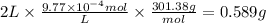Chemistry, 28.12.2019 01:31 ronniethefun
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. one tablet is dissolved in water and diluted to a volume of 2l. if the solution exhibits an absorbance of 0.687 in the uv region at 262nm in a 1- cm cell, how many grams to dobutamine are contained in the tablet? a. 0.5277g b. 0.58889 c. 9.77x 10g d. 97.778ge. 9.7778g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. o...
Questions


English, 22.10.2020 05:01



English, 22.10.2020 05:01




Mathematics, 22.10.2020 05:01





English, 22.10.2020 05:01

Computers and Technology, 22.10.2020 05:01





English, 22.10.2020 05:01