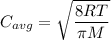Chemistry, 28.12.2019 00:31 GGerardi7552
A0.5 mol sample of he(g) and a 0.5 mol sample of ne(g) are placed separately in two 10.0 l rigid containers at 25°c. each container has a pinhole opening. which of the gases, he(g) or ne(g), will escape faster through the pinhole and why?
a) he because the he atoms are moving at a higher average speed than the ne atoms.
b) ne because its initial pressure in the container is higher
c) ne because the ne atoms have a higher average kinetic energy than the he atoms
d) both gases will escape at the same rate because the atoms of both gases have the same average kinetic enery

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
A0.5 mol sample of he(g) and a 0.5 mol sample of ne(g) are placed separately in two 10.0 l rigid con...
Questions


Mathematics, 18.01.2020 06:31

English, 18.01.2020 06:31

History, 18.01.2020 06:31


Mathematics, 18.01.2020 06:31


Mathematics, 18.01.2020 06:31

Mathematics, 18.01.2020 06:31

English, 18.01.2020 06:31




Mathematics, 18.01.2020 06:31

Mathematics, 18.01.2020 06:31





Mathematics, 18.01.2020 06:31