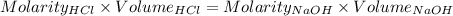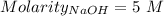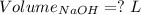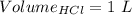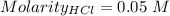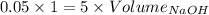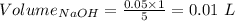Chemistry, 27.12.2019 05:31 svarner2001
Consider a solution that is 0.05 m hcl. your goal is to neutralize 1 l of this solution (i. e. bring the ph to 7). you also have a solution that is 5 m naoh. what volume of this solution should you add to the hcl solution, to neutralize it? provide your answer in units of liters (l).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
Consider a solution that is 0.05 m hcl. your goal is to neutralize 1 l of this solution (i. e. bring...
Questions



English, 25.02.2021 18:30


Mathematics, 25.02.2021 18:30



Mathematics, 25.02.2021 18:30






History, 25.02.2021 18:30



Mathematics, 25.02.2021 18:30



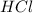 = Moles of NaOH
= Moles of NaOH