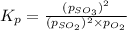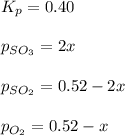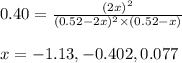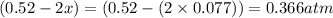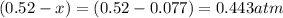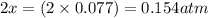Chemistry, 27.12.2019 03:31 IIHarmonyII
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g) calculate the equilibrium partial pressures of so2, o2, and so3 produced from an initial mixture in which the partial pressures of so2 and o2 = 0.52 atm and the partial pressure of so3 = 0 (exactly).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g)...
Questions




Mathematics, 04.02.2021 06:30



Mathematics, 04.02.2021 06:30


English, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30

Mathematics, 04.02.2021 06:30



History, 04.02.2021 06:30


Biology, 04.02.2021 06:30

History, 04.02.2021 06:30



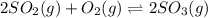
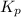 for above equation follows:
for above equation follows: