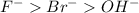Chemistry, 27.12.2019 02:31 RosaJackson8088
Consider four different samples: aqueous nabr, molten nabr, aqueous naf, and molten naf. current run through each sample produces one of the following products at the anode: liquid bromine, fluorine gas, or oxygen gas. match each sample to its anodic product.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Consider four different samples: aqueous nabr, molten nabr, aqueous naf, and molten naf. current ru...
Questions


World Languages, 21.05.2021 21:10

Biology, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10

Physics, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10

History, 21.05.2021 21:10


Biology, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10



Mathematics, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10



History, 21.05.2021 21:10

English, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10