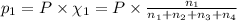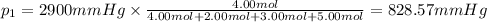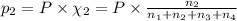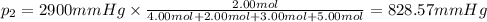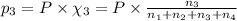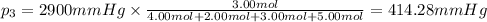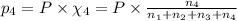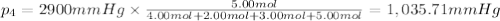Chemistry, 27.12.2019 02:31 ahmedeldyame
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles of ar. the total pressure of the mixture is 2900 mm. determine the mole fraction of each gas in the mixture. determine the mole percent of each gas in the mixture. determine the partial pressure of each gas in the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles o...
Questions


Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01




Mathematics, 22.10.2020 01:01








Mathematics, 22.10.2020 01:01


Spanish, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

English, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

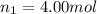
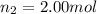
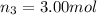
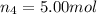
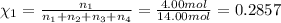
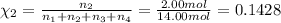
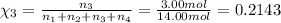
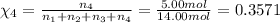
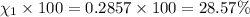
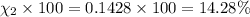
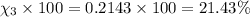
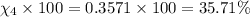
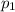
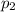
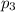
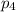
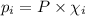
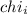 = Mole fraction of ith component
= Mole fraction of ith component