Chemistry, 27.12.2019 00:31 alexisthegirl
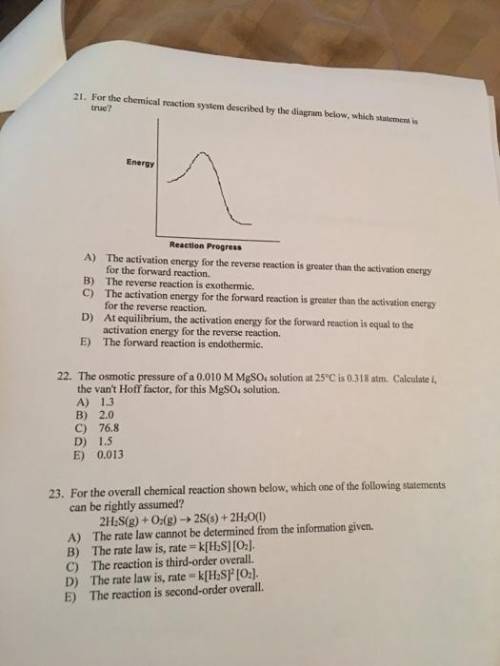
For the chemical reaction system described by the diagram below, which statement is true? picture the forward reaction is endothermic. the activation energy for the forward reaction is greater than the activation energy for the reverse reaction. at equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction. the activation energy for the reverse reaction is greater than the activation energy for the forward reaction. the reverse reaction is exothermic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
For the chemical reaction system described by the diagram below, which statement is true? picture t...
Questions

Mathematics, 12.06.2020 19:57









Mathematics, 12.06.2020 19:57




Mathematics, 12.06.2020 19:57



History, 12.06.2020 19:57

Mathematics, 12.06.2020 19:57