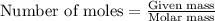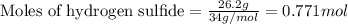Chemistry, 27.12.2019 00:31 lestessanders02
Hydrosulfuric acid (h2s) undergoes combustion to yield sulfur dioxide and water by the following reaction equation: 2 h2s + 3 o2 → 2 so2 + 2 h2o

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Hydrosulfuric acid (h2s) undergoes combustion to yield sulfur dioxide and water by the following rea...
Questions


Mathematics, 05.05.2021 07:40

Arts, 05.05.2021 07:40


Mathematics, 05.05.2021 07:40





Mathematics, 05.05.2021 07:40

Mathematics, 05.05.2021 07:40





Mathematics, 05.05.2021 07:40

English, 05.05.2021 07:40


Mathematics, 05.05.2021 07:40

Biology, 05.05.2021 07:40

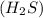 undergoes combustion to yield sulfur dioxide and water by the following reaction equation:
undergoes combustion to yield sulfur dioxide and water by the following reaction equation: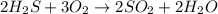
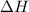 of the reaction if 26.2 g of
of the reaction if 26.2 g of  reacts with excess
reacts with excess 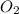 to yield 431.8 kJ?
to yield 431.8 kJ?