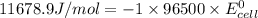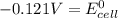Chemistry, 26.12.2019 23:31 BurwinkelElla19
Calculate the standard cell potential (e∘) for the reaction x(s)+y+(aq)→x+(aq)+y(s) if k = 8.97×10−3. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Calculate the standard cell potential (e∘) for the reaction x(s)+y+(aq)→x+(aq)+y(s) if k = 8.97×10−3...
Questions

Geography, 10.05.2021 02:20


Mathematics, 10.05.2021 02:20

Biology, 10.05.2021 02:20


Mathematics, 10.05.2021 02:20




Mathematics, 10.05.2021 02:20

Mathematics, 10.05.2021 02:20

English, 10.05.2021 02:20

Mathematics, 10.05.2021 02:20


Mathematics, 10.05.2021 02:20

Mathematics, 10.05.2021 02:20




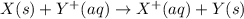 is -0.121 V
is -0.121 V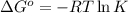
 = Standard Gibbs free energy = ?
= Standard Gibbs free energy = ?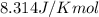
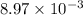
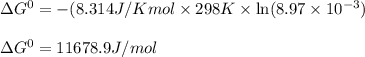
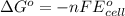
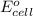 = standard cell potential = ?
= standard cell potential = ?