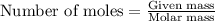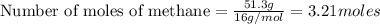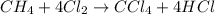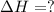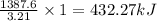Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1
You know the right answer?
Methane (ch4) reacts with cl2 to yield ccl4 and hcl by the following reaction equation: ch4 + 4 cl2...
Questions



Geography, 25.08.2019 16:30

Mathematics, 25.08.2019 16:30



Mathematics, 25.08.2019 16:30

Mathematics, 25.08.2019 16:30


Mathematics, 25.08.2019 16:30



Mathematics, 25.08.2019 16:30






Mathematics, 25.08.2019 16:30

Mathematics, 25.08.2019 16:30

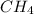 reacts with excess
reacts with excess 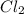 to yield 1387.6 kJ is 432.27kJ
to yield 1387.6 kJ is 432.27kJ