
Chemistry, 26.12.2019 22:31 DavidsonSaid
The acid dissociation constant ka of boric acid (h3bo3) is 5.8 times 10^-10. calculate the ph of a 4.4 m solution of boric acid. round your answer to 1 decimal place.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2


Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
The acid dissociation constant ka of boric acid (h3bo3) is 5.8 times 10^-10. calculate the ph of a 4...
Questions

History, 30.01.2021 05:30




Mathematics, 30.01.2021 05:30

History, 30.01.2021 05:30

Biology, 30.01.2021 05:30




Mathematics, 30.01.2021 05:30

Mathematics, 30.01.2021 05:30

Mathematics, 30.01.2021 05:30

Mathematics, 30.01.2021 05:30


Mathematics, 30.01.2021 05:30

Mathematics, 30.01.2021 05:30



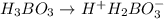
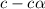
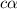
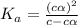
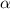 = ?
= ?
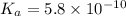
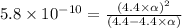
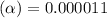
![[H^+]=c\times \alpha](/tpl/images/0434/0871/4fc41.png)
![[H^+]=4.4\times 0.000011=4.8\times 10^{-5}M](/tpl/images/0434/0871/865ab.png)
![pH=-log[H^+]](/tpl/images/0434/0871/15713.png)
![pH=-log[4.8\times 10^{-5}]=4.3](/tpl/images/0434/0871/ee066.png)
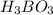 solution is 4.3
solution is 4.3

