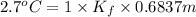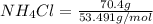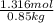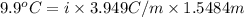When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . calculate the mass of ammonium chloride that must be dissolved in the same mass of to produce the same depression in freezing point. the van't hoff factor for ammonium chloride in .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution...
Questions

English, 11.02.2020 07:24



Mathematics, 11.02.2020 07:24



History, 11.02.2020 07:24




Mathematics, 11.02.2020 07:25


Mathematics, 11.02.2020 07:25

Social Studies, 11.02.2020 07:25

Mathematics, 11.02.2020 07:25

Mathematics, 11.02.2020 07:26

Mathematics, 11.02.2020 07:26



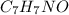 ) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is
) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is 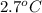 lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (
lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (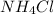 ) are dissolved in the same mass of X, the freezing point of the solution is
) are dissolved in the same mass of X, the freezing point of the solution is 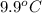 lower than the freezing point of pure X.
lower than the freezing point of pure X.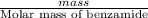
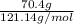
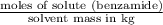
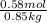
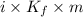 ,
, 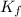 = freezing point constant of solvent
= freezing point constant of solvent