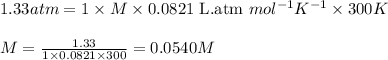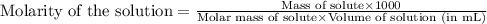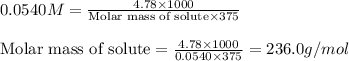Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
Asolution is prepared by dissolving 4.78 g of an unknown nonelectrolyte in enough water to make 375...
Questions

Physics, 16.10.2019 15:30


History, 16.10.2019 15:30

Arts, 16.10.2019 15:30



Biology, 16.10.2019 15:30




Mathematics, 16.10.2019 15:30

Mathematics, 16.10.2019 15:30





Physics, 16.10.2019 15:50

Geography, 16.10.2019 15:50


Mathematics, 16.10.2019 15:50

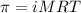
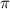 = osmotic pressure of the solution = 1.33 atm
= osmotic pressure of the solution = 1.33 atm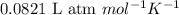
![27^oC=[273+27]K=300K](/tpl/images/0434/0272/00f96.png)