
Chemistry, 26.12.2019 21:31 brandonthomas11
A2.5 l flask is filled with 0.25 atm so3, 0.20 atm so2, and 0.40 atm o2, and allowed to reach equilibrium. assume at the temperature of the mixture is chosen so that kp = 0.12. predict the effect on the partial pressure of so3 as equilibrium is achieved by using q, the reaction quotient.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
A2.5 l flask is filled with 0.25 atm so3, 0.20 atm so2, and 0.40 atm o2, and allowed to reach equili...
Questions


History, 03.08.2019 02:40


History, 03.08.2019 02:40

English, 03.08.2019 02:40



Spanish, 03.08.2019 02:40




Mathematics, 03.08.2019 02:40




English, 03.08.2019 02:40

English, 03.08.2019 02:40


English, 03.08.2019 02:40

Arts, 03.08.2019 02:40

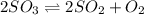
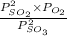
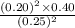
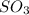 as equilibrium is achieved by using Q, is as follows.
as equilibrium is achieved by using Q, is as follows.

