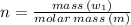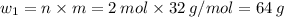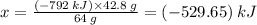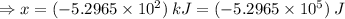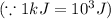Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
Sulfur undergoes combustion to yield sulfur trioxide by the following reaction equation:
Questions


Biology, 05.05.2020 04:56

English, 05.05.2020 04:56


English, 05.05.2020 04:56


Biology, 05.05.2020 04:56


Chemistry, 05.05.2020 04:56


Mathematics, 05.05.2020 04:56


Mathematics, 05.05.2020 04:56




Mathematics, 05.05.2020 04:56


Social Studies, 05.05.2020 04:56

Mathematics, 05.05.2020 04:56