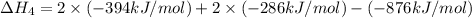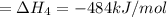Chemistry, 25.12.2019 04:31 smokemicpot
Alculate the standard enthalpy of formation of ethanoic acid given that the standard enthalpy of combustion for carbon is –394 kj mol-1 , hydrogen is –286 kj mol-1 and ethanoic acid is –876 kj mol-

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Alculate the standard enthalpy of formation of ethanoic acid given that the standard enthalpy of com...
Questions

Social Studies, 27.09.2019 11:10


Physics, 27.09.2019 11:10


Mathematics, 27.09.2019 11:10

Mathematics, 27.09.2019 11:10

History, 27.09.2019 11:10

History, 27.09.2019 11:10



Chemistry, 27.09.2019 11:10

Mathematics, 27.09.2019 11:10

Mathematics, 27.09.2019 11:10

Computers and Technology, 27.09.2019 11:10

Social Studies, 27.09.2019 11:10


Biology, 27.09.2019 11:10



Mathematics, 27.09.2019 11:10

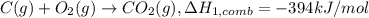 ...[1]
...[1]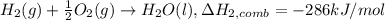 ..[2]
..[2]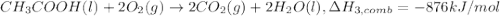 ..[3]
..[3]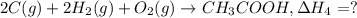 ..[4]
..[4]