
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285 g) and h20 (0.658g) are produced. the mol mass of the compound is determined by mass spectrometry to be 90 g/mole determine the empirical and molecular formulas. 20: 0-8453x(12.01s /uu. o 032 029/1b. a mal 1 2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 13:20
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1

Chemistry, 23.06.2019 18:30
Which of the following describes geothermal energy in use
Answers: 1
You know the right answer?
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285...
Questions

English, 13.09.2020 02:01

Biology, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

History, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

English, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

Mathematics, 13.09.2020 02:01

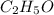
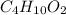
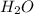 = 0.658 g /18 g/mol = 0.03656 moles
= 0.658 g /18 g/mol = 0.03656 moles
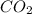 = 1.285 g
/44.01 g/mol = 0.029197 moles
= 1.285 g
/44.01 g/mol = 0.029197 moles


