
Chemistry, 24.12.2019 22:31 morgan4411
Apotential source of error for this lab could arise if not enough acid was added to the iron solution before the titration was started. this would allow much of permanganate to go to mno2 rather then mn 2 . how would this affect the % of iron found at the end of the experiment

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2
You know the right answer?
Apotential source of error for this lab could arise if not enough acid was added to the iron solutio...
Questions

Social Studies, 26.09.2019 01:20

Biology, 26.09.2019 01:20


Mathematics, 26.09.2019 01:20


Mathematics, 26.09.2019 01:20

History, 26.09.2019 01:20




History, 26.09.2019 01:20

History, 26.09.2019 01:20

History, 26.09.2019 01:20

Mathematics, 26.09.2019 01:20



Biology, 26.09.2019 01:20



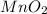 is produced instead of manganese (II) ions,
is produced instead of manganese (II) ions, 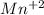 , such oxide will act as a contaminant in the final product collection, affecting the experiment reliability when finding the iron, that is why we must assure the total reduction to manganese (II) ions.
, such oxide will act as a contaminant in the final product collection, affecting the experiment reliability when finding the iron, that is why we must assure the total reduction to manganese (II) ions.

