Chemistry, 24.12.2019 20:31 smartgirl61987
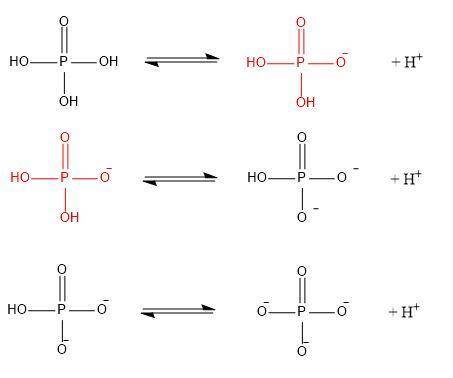
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15, 7.20, and 12.35 respectively.
a/ write out the series of ionization (equilibrium) reactions corresponding to each ionization, making sure to write out the (flat) structure each molecule/ion as you do so. mark correct chemical bonds.
b/ in your diagram above, circle the dominant form of phosphate as it would appear at ph 5.7.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1



You know the right answer?
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15...
Questions

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

English, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

English, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

English, 17.09.2020 21:01

Geography, 17.09.2020 21:01

English, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

History, 17.09.2020 21:01