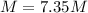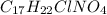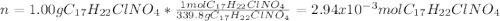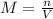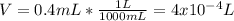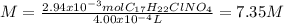Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 13:00
Aecosystem is if it can continue to function over long periods of time
Answers: 1
You know the right answer?
The hydrochloride form of cocaine has a solubility of 1.00 g in 0.400 ml water. calculate the molari...
Questions


Mathematics, 22.10.2020 23:01



SAT, 22.10.2020 23:01

History, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01



Chemistry, 22.10.2020 23:01

English, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01



Mathematics, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01