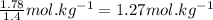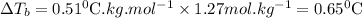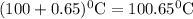Chemistry, 24.12.2019 20:31 tracyaleblanc
What is the boiling point of a solution produced by adding 610 g of cane sugar (molar mass 342.3 g/mol) to 1.4 kg of water? for each mole of nonvolatile solute, the boiling point of 1 kg of water is raised 0.51 ∘c.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
What is the boiling point of a solution produced by adding 610 g of cane sugar (molar mass 342.3 g/m...
Questions



Mathematics, 19.09.2021 05:00


Mathematics, 19.09.2021 05:00

Mathematics, 19.09.2021 05:00

Chemistry, 19.09.2021 05:00

Biology, 19.09.2021 05:00

Computers and Technology, 19.09.2021 05:00


Mathematics, 19.09.2021 05:00



Mathematics, 19.09.2021 05:00






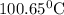
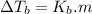
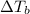 is elivation in boiling point of solution,
is elivation in boiling point of solution, 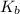 is ebbulioscopic constant of solvent (how much temperature is raised for dissolution of 1 mol of non-volatile solute) and m is molality of solution.
is ebbulioscopic constant of solvent (how much temperature is raised for dissolution of 1 mol of non-volatile solute) and m is molality of solution.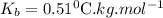
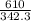 moles of cane sugar
moles of cane sugar