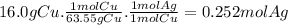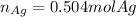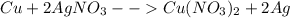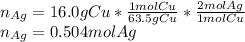Given the following reaction: \ce{cu + 2agno3 -> 2ag + cu(no3)2}cu+2agno3 2ag+cu(nox 3 )x 2 how many moles of \ce{ag}aga, g will be produced from 16.0 \text{ g}16.0 g16, point, 0, start text, space, g, end text of \ce{cu}cuc, u, assuming \ce{agno3}agno3 is available in excess

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

You know the right answer?
Given the following reaction: \ce{cu + 2agno3 -> 2ag + cu(no3)2}cu+2agno3 2ag+cu(nox 3 )x 2...
Questions




Mathematics, 26.05.2021 22:40


Chemistry, 26.05.2021 22:40

SAT, 26.05.2021 22:40







Mathematics, 26.05.2021 22:40




Mathematics, 26.05.2021 22:40

Mathematics, 26.05.2021 22:40