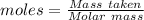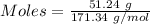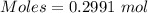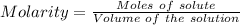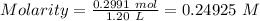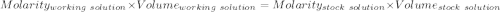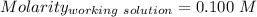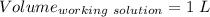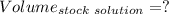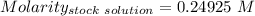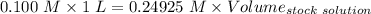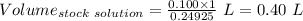Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
A51.24-g sample of ba(oh)2 is dissolved in enough water to make 1.20 liters of solution. how many ml...
Questions

Social Studies, 04.02.2021 14:00

Biology, 04.02.2021 14:00

Chemistry, 04.02.2021 14:00


Mathematics, 04.02.2021 14:00

Geography, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00




English, 04.02.2021 14:00

Physics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Physics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00


Advanced Placement (AP), 04.02.2021 14:00

Social Studies, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

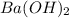 as:-
as:-