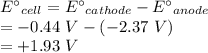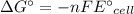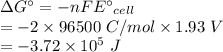Calculate the standard free-energy change at 25 ∘c for the following reaction:
mg(s)+fe2+(aq...

Chemistry, 24.12.2019 04:31 okasiafolk27
Calculate the standard free-energy change at 25 ∘c for the following reaction:
mg(s)+fe2+(aq)→mg2+(aq)+fe(s)
express your answer to three significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Questions


Mathematics, 10.11.2020 05:50

Mathematics, 10.11.2020 05:50

English, 10.11.2020 05:50



Mathematics, 10.11.2020 05:50

Biology, 10.11.2020 05:50

English, 10.11.2020 05:50


Mathematics, 10.11.2020 05:50





Mathematics, 10.11.2020 05:50


Spanish, 10.11.2020 05:50

Chemistry, 10.11.2020 05:50

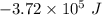
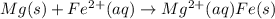
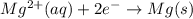 E° = -2.37 V
E° = -2.37 V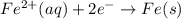 E° = -0.44 V
E° = -0.44 V