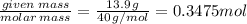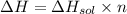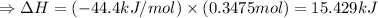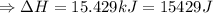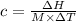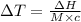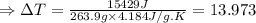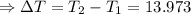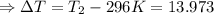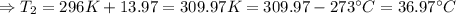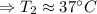Chemistry, 24.12.2019 02:31 coolman5999alt
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves in 250.0 g of water 23.0 °c in a coffee-cup calorimeter, what is the final temperature of the solution assuming no heat is lost to the surroundings. the solution has the same specific heat of 4.184 j/g-k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2

Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3
You know the right answer?
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves...
Questions

History, 21.12.2020 14:00

Engineering, 21.12.2020 14:00



Mathematics, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00


Chemistry, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00



Mathematics, 21.12.2020 14:00



English, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00



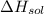 = – 44.4 kJ/mol,
= – 44.4 kJ/mol,