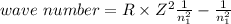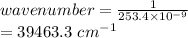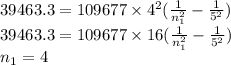Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1


Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
One of the emission spectral lines for be3+ has a wavelength of 253.4 nm for an electronic transitio...
Questions


Computers and Technology, 07.01.2020 05:31

Computers and Technology, 07.01.2020 05:31

History, 07.01.2020 05:31


English, 07.01.2020 05:31




History, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

English, 07.01.2020 05:31


Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31


Mathematics, 07.01.2020 05:31