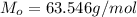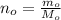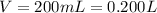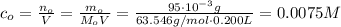Chemistry, 23.12.2019 22:31 eweqwoewoji
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: fe(s)+cuso4(aq)→cu(s)+feso4(aq)supp ose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a 200.ml copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of 95.mg. calculate the original concentration of copper(ii) sulfate in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which con...
Questions





English, 29.07.2019 01:40




Mathematics, 29.07.2019 01:40

Mathematics, 29.07.2019 01:40

Mathematics, 29.07.2019 01:40




Mathematics, 29.07.2019 01:40



Mathematics, 29.07.2019 01:40

Computers and Technology, 29.07.2019 01:40