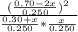Suppose a 250. ml flask is filled with 0.30 mol of n_2 and 0.70 mol of no. the following reaction becomes possible: n_2(g) +o2 → 2no(g)a. the equilibrium constant k for this reaction is 7.70 at the temperature of the flask. b. calculate the equilibrium molarity of o2. round your answer to one decimal place.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
Suppose a 250. ml flask is filled with 0.30 mol of n_2 and 0.70 mol of no. the following reaction be...
Questions


History, 23.11.2020 07:20


Health, 23.11.2020 07:30


Mathematics, 23.11.2020 07:30



Mathematics, 23.11.2020 07:30



Social Studies, 23.11.2020 07:30

Computers and Technology, 23.11.2020 07:30

History, 23.11.2020 07:30

Chemistry, 23.11.2020 07:30

Mathematics, 23.11.2020 07:30


Mathematics, 23.11.2020 07:30


Mathematics, 23.11.2020 07:30