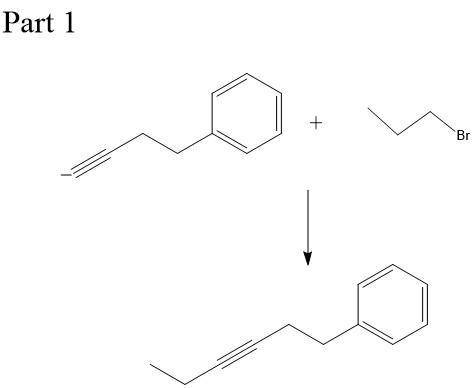
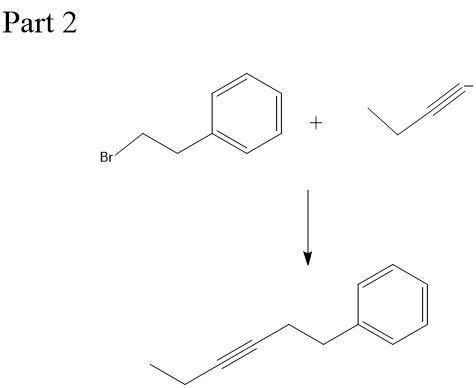
Chemistry, 23.12.2019 21:31 naomicervero
This molecule can be synthesized from an alkyne anion and an alkyl bromide. however, there are two ways in which this molecule can be formed. one way uses a higher molecular weight alkyne anion (part 1) and the other uses a lower molecular weight anion (part 2). draw the two versions in the boxes below. omit spectator ions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


You know the right answer?
This molecule can be synthesized from an alkyne anion and an alkyl bromide. however, there are two w...
Questions




Mathematics, 28.07.2021 03:30


Mathematics, 28.07.2021 03:30

Business, 28.07.2021 03:30









Chemistry, 28.07.2021 03:30

Mathematics, 28.07.2021 03:30



Mathematics, 28.07.2021 03:30