
Chemistry, 23.12.2019 20:31 serenityarts123
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 l.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate...
Questions





Business, 24.07.2021 21:40

Computers and Technology, 24.07.2021 21:40

Arts, 24.07.2021 21:40

Mathematics, 24.07.2021 21:40

Mathematics, 24.07.2021 21:40


Mathematics, 24.07.2021 21:40

Mathematics, 24.07.2021 21:40


Mathematics, 24.07.2021 21:40

Mathematics, 24.07.2021 21:40

English, 24.07.2021 21:40

English, 24.07.2021 21:40

Mathematics, 24.07.2021 21:40


English, 24.07.2021 21:40

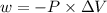
 is the change in volume
is the change in volume

 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)

