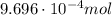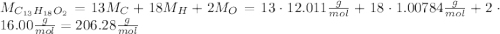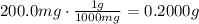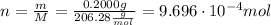Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1


Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3
You know the right answer?
ibuprofen (c13h1802) is the active ingredient in many nonprescription pain relievers. each tablet co...
Questions




Biology, 22.08.2019 22:30

Mathematics, 22.08.2019 22:30




English, 22.08.2019 22:30


Mathematics, 22.08.2019 22:30





English, 22.08.2019 22:30

History, 22.08.2019 22:30