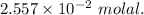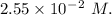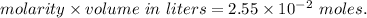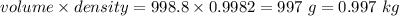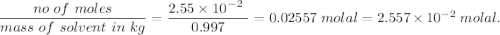Chemistry, 21.12.2019 06:31 genyjoannerubiera
A2.550 x 10^−2 m solution of glycerol (c3h8o3) in water is at 20.0°c. the sample was created by dissolving a sample of c3h8o3 in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 998.9 ml . the density of water at 20.0°c is 0.9982 g/ml.
a. calculate the molality of the glycerol solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
A2.550 x 10^−2 m solution of glycerol (c3h8o3) in water is at 20.0°c. the sample was created by diss...
Questions


Mathematics, 18.10.2020 08:01

Biology, 18.10.2020 08:01


Mathematics, 18.10.2020 08:01

Biology, 18.10.2020 08:01



English, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01

Social Studies, 18.10.2020 08:01

Biology, 18.10.2020 08:01


Mathematics, 18.10.2020 08:01

Health, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01




World Languages, 18.10.2020 08:01