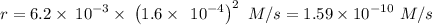The second-order rate constant for the dimerization of a protein (p) p + p → p2 is 6.2 × 10−3/m · s at 25°c. part 1 out of 2 if the concentration of the protein is 1.6 × 10−4 m, calculate the initial rate (m/s) of formation of p2. rate = × 10 m/s (enter your answer in scientific notation.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
The second-order rate constant for the dimerization of a protein (p) p + p → p2 is 6.2 × 10−3/m · s...
Questions

Biology, 27.08.2019 20:30





Mathematics, 27.08.2019 20:30



Mathematics, 27.08.2019 20:30




History, 27.08.2019 20:30

Mathematics, 27.08.2019 20:30

History, 27.08.2019 20:30




History, 27.08.2019 20:30

Geography, 27.08.2019 20:30

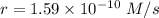
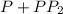
![r=k[P]^2](/tpl/images/0428/7363/45884.png)
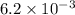 /Ms
/Ms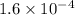 M
M