Chemistry, 21.12.2019 04:31 MrRandomUser
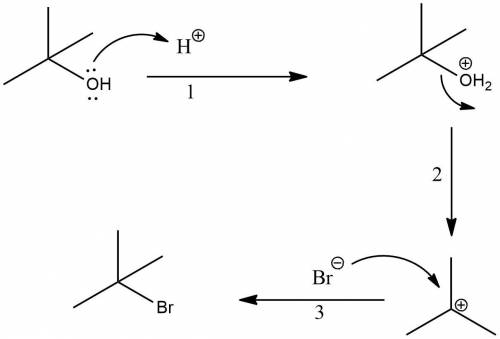
T-butyl bromide (2-bromo-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking it with an aqueous solution of hbr at room temperature. the reaction is much faster than with n-butyl alcohol and is essentially 100% complete within a few minutes. give a mechanism for this reaction. what is this type of reaction called

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
T-butyl bromide (2-bromo-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaki...
Questions

Mathematics, 22.01.2021 01:10


Mathematics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10


Physics, 22.01.2021 01:10


Mathematics, 22.01.2021 01:10

Arts, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10





Physics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10

Mathematics, 22.01.2021 01:10

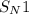 (substitution nucleophilic bimolecular) reaction.
(substitution nucleophilic bimolecular) reaction.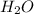 .
.