
Chemistry, 21.12.2019 00:31 wolfgirl2431
Achemical reaction turns substance a into substance b. the rate at which the reaction takes place is proportional to the square root of the amount of substance a present.
if is the amount of substance a present (measured in grams) at any time (measured in minutes), set up a differential equation for in terms of . if there is an arbitrary constant in your equation, call it and make sure that > 0k> 0.
now, suppose that the chemical reaction is to take place in a large tank of liquid!
the tank contains 25 liters of a solution of substance a dissolved in water. the concentration of the solution is 750 grams per liter. a 200 gram per liter solution of substance a is poured in at 12 liters per minute. at the same time, the solution is drained from the bottom of the tank at 11 liters per minute.
if is the amount of substance a in the tank (measured in grams) at any time (measured in minutes), set up a differential equation for in terms of :

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Achemical reaction turns substance a into substance b. the rate at which the reaction takes place is...
Questions


Mathematics, 28.11.2019 05:31













Computers and Technology, 28.11.2019 05:31





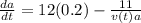
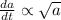
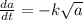
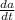 will be high
will be high
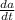 = 12 (200 grams) - 11/(v(t))a
= 12 (200 grams) - 11/(v(t))a


