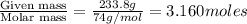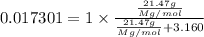Chemistry, 20.12.2019 21:31 cindy14772
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. the vapor pressure of diethyl ether, ch3ch2och2ch3, is 463.57 mm hg at 25°c.
1. in a laboratory experiment, students synthesized a new compound and found that when 21.47 grams of the compound were dissolved in 233.8 grams of diethyl ether, the vapor pressure of the solution was 455.55 mm hg. the compound was also found to be nonvolatile and a non-electrolyte. what is the molecular weight of this compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in...
Questions


Physics, 11.11.2020 03:50



History, 11.11.2020 03:50



History, 11.11.2020 03:50




Computers and Technology, 11.11.2020 03:50

Mathematics, 11.11.2020 03:50

English, 11.11.2020 03:50

Mathematics, 11.11.2020 03:50


Chemistry, 11.11.2020 03:50

Mathematics, 11.11.2020 03:50


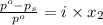
 = relative lowering in vapor pressure
= relative lowering in vapor pressure = mole fraction of solute =
= mole fraction of solute =